Two containers of equal volume are connected by a stopcock as shown below. One container is filled with a gas at a pressure of 1 atm and temperature of 293 K while the other container is evacuated so that it is under vacuum. The containers are thermally isolated from the surrounding so no heat enters or escaped from the system. The stopcock is then opened allowing the gas from one container to fill the other. What is the final temperature of the gas after it has come to equilibrium? 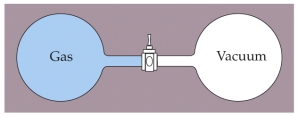
Definitions:
Frightened Infant
A term not typically used in professional discourse, possibly indicating an infant experiencing fear or distress.
Securely Attached
Describes individuals who have healthy, trusting, and resilient relationships, often stemming from consistent caregiving in infancy.
Strange Situation
A standardized procedure devised by Mary Ainsworth to observe attachment relationships between a caregiver and children.
Facial Expression
The movement and configuration of facial muscles to convey emotions or reactions without verbal communication.
Q6: What is the major product for the
Q37: The energy of a simple harmonic oscillator
Q47: The molar specific heat of copper
Q87: What is the major product of the
Q100: A longitudinal wave is distinguished from a
Q112: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6080/.jpg" alt=" A pulse moves
Q113: Which of the following species contributes more
Q116: A jet engine emits a whine of
Q157: What is the major product of the
Q161: Which of the following could be used