Use the following to answer the question: 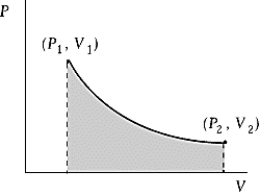
-An ideal gas initially at 100ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much does the internal energy of the gas change during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
Definitions:
Manufacturing
The process of producing goods and products in large quantities through the use of labor, machines, tools, and chemical or biological processing.
Robotic Pizza Delivery
The use of autonomous robots to deliver pizzas from the restaurant to the customer's location.
Substitute Products
Substitute products are goods or services that can serve as replacements for each other, fulfilling the same need or purpose, and thus, are in competition with one another.
Rivalry
Describes competition or conflict between individuals, organizations, or nations, often driving innovation or improvement.
Q4: A stretched string of length L, fixed
Q8: The number of unique open-chain structures corresponding
Q27: A traveling wave passes a point of
Q30: A container has 0.2 mole of
Q47: The molar specific heat of copper
Q67: The temperature of the air on a
Q71: If the heat given off by 300
Q74: When driving over a washboard speed bumps,
Q75: When you push a child in a
Q90: Two waveforms of the same frequency are