Use the following to answer the question: 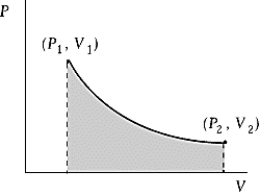
-An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 50 kPa.How much does the internal energy of the gas change during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
Definitions:
Psychoanalytic Theory
A theory of human development that contends that irrational, unconscious drives and motives underlie human behavior.
Moral Standards
Principles or rules of behavior based on ideas about what is morally good or bad, guiding ethical judgment and actions.
Personality
A person's special mix of emotional, attitudinal, and behavioral reactions.
Cross-Cultural Research
Studies designed to compare and contrast cultures, exploring how values, behaviors, and norms differ and are the same across societies.
Q14: What alkyl groups make up the following
Q16: How many compounds are possible from the
Q33: In which molecule(s)can the molecular geometry be
Q65: If a mass of oxygen gas occupies
Q68: A siren of frequency 3.20 kHz moves
Q74: A noisy workplace has a noise level
Q99: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6080/.jpg" alt=" The work done
Q106: To double the period of a pendulum,
Q117: Draw Fischer projection formula(s)of the major product(s)of
Q137: Which molecule(s)has/have dipole moment(s)equal to zero?<br>A) <img