Use the following diagram to answer the next problem. 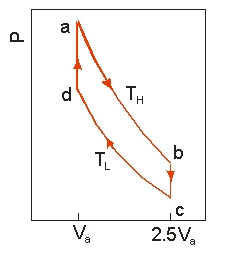 An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-How much work is obtained from the engine in each cycle?
Definitions:
Q9: The graph shows the average power delivered
Q20: When the 1s orbitals of two hydrogen
Q33: The air around us has 78% nitrogen
Q45: A common trick to open the
Q49: What is the major product of the
Q57: A box contains 10 red and 10
Q62: The interaction of the <span
Q65: What is the major product of the
Q122: The bond that results when two atoms
Q136: Drawn below is atropine,found in Atropa belladonna,sometimes