The thermodynamic parameters at 298 K for the following reaction are given below. 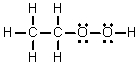
Hº = -64.9 kJ mol-1 Which of the following statements is true of the reaction?
Definitions:
Geometric Sequence
A numerical series where each number after the first is determined by multiplying the prior number by an unchanging, non-zero numerical value, denoted as the common ratio.
Partial Sum
A partial sum is the sum of the first n terms of a sequence, often used in the context of series to understand the progression or to investigate convergence.
N Th
In mathematics, "n-th" refers to an unspecified member of a sequence, indicating its position with the variable n.
Geometric Sequence
A progression of numbers in which each term beyond the initial one is produced by the multiplication of the preceding term by a constant, non-zero multiplier called the common ratio.
Q16: In addition to a cycloalkane skeleton,testosterone also
Q24: You can double both the pressure and
Q48: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6080/.jpg" alt=" The graph shows
Q68: A temperature of 14ºF is equivalent to<br>A)
Q70: A device used to measure the
Q79: Which of these is not a Lewis
Q105: If a sound of intensity I
Q113: What is the major product of the
Q114: What functional groups are present in nicotine,an
Q138: A reagent or test that could be