The thermodynamic parameters at 298 K for the following reaction are given below. 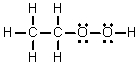
Hº = -64.9 kJ mol-1 Which of the following statements is true of the reaction?
Definitions:
Native American
Refers to the indigenous peoples and cultures of the United States, including their history, traditions, and present-day communities.
Immigrant Families
Families that have relocated from their country of origin to another country, often facing unique social, economic, and cultural challenges.
Learning Problems
Difficulties in acquiring knowledge or skills to the expected level, often requiring specialized interventions.
Communication Skills
Communication skills involve the ability to convey or share ideas and feelings effectively, including listening, speaking, reading, and writing.
Q17: An unknown compound X was subjected to
Q20: A steam power plant with an efficiency
Q22: One mole of an ideal gas
Q26: Which of the following statements about the
Q68: In a system composed of gas contained
Q76: A vibrating tuning fork of 300
Q90: The quantity of heat absorbed by a
Q92: Considering Lewis structures,which of these compounds possesses
Q112: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6080/.jpg" alt=" A pulse moves
Q150: What functional group is present in the