For the Following Acid-Base Reaction,which Statement Is True Taking S into Consideration?
A)The Reaction Is an Exothermic
For the following acid-base reaction,which statement is true taking S into consideration? 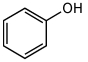
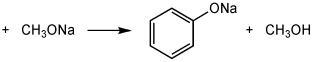
Definitions:
Q4: The strongest acid that can exist in
Q25: I and II are: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg" alt="I
Q54: What is the major product for the
Q59: Write an equation to show the reaction
Q69: Which compound does NOT possess a plane
Q85: Which of the following molecules or ions
Q102: Which compound would you expect to have
Q112: A conjugate base of sulfuric acid is:<br>A)H<sub>3</sub>SO<sub>4</sub><sup>+</sup><br>B)SO<sub>3</sub><br>C)HSO<sub>4</sub><sup>-</sup><br>D)H<sub>2</sub>SO<sub>3</sub><br>E)HSO<sub>3</sub><sup>-</sup>
Q126: Ozonolysis of compound Z yields the products
Q170: Cis-trans isomers are:<br>A)diastereomers.<br>B)enantiomers.<br>C)stereoisomers.<br>D)constitutional isomers.<br>E)More than one of