For the following acid/base reaction which statement is true taking S into consideration? 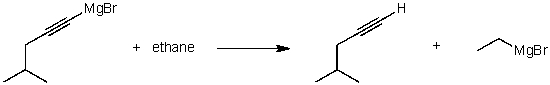
Definitions:
Policy Tasks
Specific actions or responsibilities that are carried out in order to implement or enforce policies.
Empathy
The ability to understand and share the feelings of another, fostering a sense of shared experience or compassion.
Sympathy
Feelings of pity and sorrow for someone else's misfortune.
Buffering
The technique of starting a message with neutral or positive information before delivering bad news or sensitive information.
Q8: Which of the structures below would be
Q26: Which of the following can be described
Q60: I and II are: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg" alt="I
Q72: Carbon and other elements in the second
Q86: What functional group is present in the
Q94: What is/are the product(s)of the following acid-base
Q97: Which of the following species is a
Q127: Consider the following: CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH=CHCH<sub>2</sub>CH<sub>2</sub>CH<sub>3 </sub><sub> </sub><sub> </sub>CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH=CH<sub>2</sub><br>I
Q143: The molecules below are: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg" alt="The
Q171: Enantiomers are:<br>A)molecules that have a mirror image.<br>B)molecules