For the following acid / base reaction which statement is true taking H into consideration? 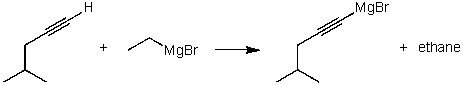
Definitions:
Anaerobes
Microorganisms having the ability to live without oxygen.
Ectoparasites
Organisms that live on the external surface of a host, such as ticks and lice, surviving by feeding on the host's blood or skin.
Obligate Parasites
Organisms that cannot complete their life-cycle without exploiting a suitable host.
Steam Pressure
The pressure exerted by steam, especially in steam engines or cooking devices, often measured in pounds per square inch (psi) or bar.
Q23: Which reaction of these potential acids and
Q23: What is the index of hydrogen deficiency
Q36: Which reaction must take place with retention
Q44: Give the IUPAC name corresponding to the
Q79: A chiral molecule was found to have
Q85: Which of the following molecules or ions
Q90: What is the correct IUPAC name for
Q93: What is the complete IUPAC name of
Q127: For the following acid-base reaction,which statement
Q128: When discussing the stereochemical relationship of cis/trans