For the following acid/base reaction which statement is true taking S into consideration? 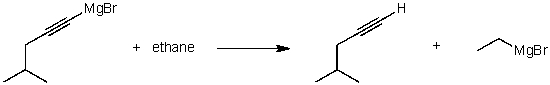
Definitions:
Compensatory
Relating to actions or mechanisms aimed at counterbalancing or making up for a deficiency or loss.
Conjunctive
Relating to connections or associations between ideas, conditions, or events, emphasizing the co-occurrence and collaboration of factors.
Disjunctive
Pertaining to a relationship or situation involving a choice between mutually exclusive options.
Free Rider
An individual who benefits from resources or services without contributing to their cost or maintenance.
Q5: How many s-sp<sup>3</sup> bonds are there in
Q18: The drug Crixivan (an HIV protease inhibitor)depicted
Q30: Which base would most effectively deprotonate benzoic
Q71: Which reaction will yield CH<sub>3</sub>CH<sub>2</sub>-D?<br>A)CH<sub>3</sub>CH<sub>3</sub> + D<sub>2</sub>O<br>B)CH<sub>3</sub>CH<sub>2</sub>Li
Q73: The bond angle for the H-C-O
Q101: According to Lewis theory,a base is a
Q126: A racemic mixture shows a specific rotation
Q133: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg" alt=" Which of the
Q135: <span class="ql-formula" data-value="\pi"><span class="katex"><span class="katex-mathml"><math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow><mi>π</mi></mrow><annotation encoding="application/x-tex">\pi</annotation></semantics></math></span><span
Q174: The molecules below are: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg" alt="The