For the following acid/base reaction which statement is true taking S into consideration? 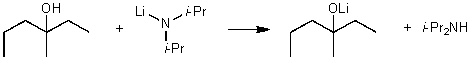
Definitions:
Lungfish
A type of freshwater fish with the unique ability to breathe air using lungs, allowing them to survive in habitats with low oxygen levels.
Lobe-Finned Fishes
A class of fish known for their fleshy, lobed, paired fins, which are precursors to the limbs of land-dwelling vertebrates.
Amphibians
A class of cold-blooded vertebrates that includes frogs, toads, salamanders, and newts, which typically have a lifecycle divided between aquatic larval stages and terrestrial adult stages.
Reptiles
A class of cold-blooded, scaly vertebrates that breathe air through lungs, including snakes, lizards, turtles, and crocodiles.
Q7: When the 1s orbitals of two hydrogen
Q41: Adding sodium hydride,NaH,to water produces:<br>A)H<sub>2</sub> and NaOH(aq)<br>B)H<sup>-</sup>(aq)+
Q42: As we go down Group 7A of
Q101: How many s-sp<sup>2</sup> bonds are there in
Q129: Which of the following species contributes more
Q144: Pairs of enantiomers are: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg" alt="Pairs
Q145: Which molecule has a plane of symmetry?
Q149: What is the name of the IUPAC
Q160: The compounds whose molecules are shown below
Q161: What is the index of hydrogen deficiency