When the following substance is treated with Br2/FeBr3,the major product is obtained in good yield,and only very small amounts of minor products are found.What is this major product and why are the minor products not formed to any significant degree? Explain clearly. 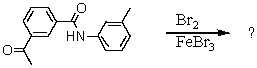
Definitions:
Concentration Ratio
A measure used in economics to assess the extent of market control by the top firms in an industry.
Oligopolies
A market structure characterized by a small number of large firms that dominate the market, often leading to limited competition and higher prices.
Collusion
An agreement, often illegal, between firms to limit competition by fixing prices, dividing markets, or coordinating production.
Concentration Ratio
A measure of the total output produced in an industry by a given number of firms, usually the largest in the industry, indicating the degree of market concentration.
Q6: The major product(s),A,of the following reaction, <img
Q16: Which compound contains the most acidic hydrogens?<br>A)CH<sub>3</sub>CH<sub>2</sub>CH<sub>3</sub><br>B)CH<sub>3</sub>CH=CH<sub>2</sub><br>C)Cyclohexane<br>D)(CH<sub>3</sub>)<sub>2</sub>C=O<br>E)Benzene
Q21: Which compound would be least reactive toward
Q49: What would be the major product(s)of the
Q50: Predict the major organic product of the
Q80: Which of the following compounds would be
Q135: Carbonation of a Grignard reagent or the
Q151: Which of the following compounds would yield
Q172: What would be the major product of
Q175: What is the expected product,A,of the following