Walp Corporation's most recent balance sheet and income statement appear below: 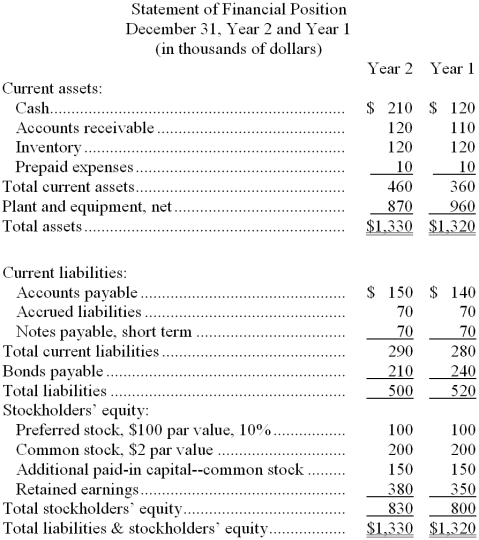

Dividends on common stock during Year 2 totaled $20 thousand. Dividends on preferred stock totaled $10 thousand. The market price of common stock at the end of Year 2 was $7.75 per share.
Required:
Compute the following for Year 2:
a. Gross margin percentage.
b. Earnings per share (of common stock).
c. Price-earnings ratio.
d. Dividend payout ratio.
e. Dividend yield ratio.
f. Return on total assets.
g. Return on common stockholders' equity.
h. Book value per share.
Definitions:
Element Cl
Chlorine, a reactive halogen element found in the periodic table with the symbol Cl, used in various industrial and chemical processes.
Halite
A mineral better known as rock salt, formed by the evaporation of salty water in ancient seas or lakes.
Element F
Fluorine, a highly reactive and electronegative element in the halogen group with the symbol F.
Silicon Tetrahedron
A fundamental structural unit of silicate minerals, consisting of four oxygen atoms surrounding a central silicon atom in a tetrahedral shape.
Q2: An increase in the bonds payable account
Q13: Below is a time series graph for
Q23: The probability that a consumer does not
Q24: Andreoli Corporation's most recent balance sheet appears
Q25: If a distribution for a quantitative variable
Q38: When using the indirect method to prepare
Q49: A properly constructed segmented income statement in
Q55: (Ignore income taxes in this problem.) The
Q98: What is the net monetary advantage (disadvantage)
Q195: What is Poblano's price-earnings ratio for last