For the following question(s) ,consider the following balanced equation. Mg3N2+ 6H2O → 3Mg(OH) 2 + 2NH3
-How many grams of 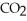 are produced from 125 g of
are produced from 125 g of 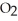 and excess
and excess 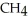 ?
?  + 2
+ 2 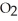 →
→ 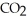 + 2
+ 2 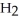 O
O
Definitions:
Sample Mean
The average of a set of numerical values drawn from a larger population, often used to estimate the population mean.
Population
The entire pool from which a sample is drawn and that is the subject of statistical analysis.
Sample Mean
The average value found from a sample, which is a subset of a larger population.
Population
The entire group of individuals or instances about whom the study is focused, from which a sample may be drawn.
Q14: Adjustment of children with chronic illness is
Q17: Successful treatment of children who have experienced
Q19: A value of 36 mL is a
Q29: What is the formula of carbon tetraiodide?<br>A)CI<br>B)
Q39: The nuclear symbol that completes the equation
Q78: The shape of the ammonia molecule (NH3)is
Q78: One mole of neon atoms has a
Q80: Which of the following properties is not
Q84: The common unit of radioactivity which is
Q85: The degree Celsius is a unit of