For the following question(s) ,consider the following balanced equation. Mg3N2+ 6H2O → 3Mg(OH) 2 + 2NH3
-How many grams of 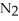 are produced when 125 g of
are produced when 125 g of 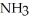 reacts as shown in the following balanced equation? 4
reacts as shown in the following balanced equation? 4 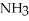 + 6NO → 5
+ 6NO → 5 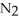 + 6
+ 6 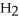 O
O
Definitions:
Automatic Transmission Fluid
A type of oil specially formulated for use in automatic transmissions to lubricate, clean, and cool internal components.
General Purpose
Designed for a variety of uses or tasks rather than specialized functions, applicable to many situations.
Parallelogram-type
Typically refers to a mechanism or structure whose opposite sides are parallel and equal in length, often used in steering systems to maintain wheel alignment.
Control Arm
A suspension component in a vehicle that connects the chassis to the wheel hub, allowing for motion while also maintaining alignment.
Q4: As a solid melts,its temperature does not
Q11: Iron rusting is a chemical change.
Q12: A kilocalorie of heat is required to
Q12: By the age of _,the brain has
Q30: In comparison to the restricting type,individuals with
Q33: A patient receives 4 × <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg"
Q71: The metallic character of elements _.<br>A)decreases going
Q80: Which of the following properties is not
Q100: The name of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="The name
Q100: How many moles of water, H<sub>2</sub>O,are present