For the following question(s) ,consider the following balanced equation. Mg3N2+ 6H2O → 3Mg(OH) 2 + 2NH3
-How many grams of 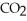 are produced from 125 g of
are produced from 125 g of 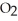 and excess
and excess 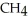 ?
?  + 2
+ 2 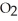 →
→ 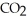 + 2
+ 2 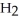 O
O
Definitions:
Research Methodology
The systematic plan, approach, and procedures for conducting scientific research to ensure valid and reliable results.
Philosophy
The study of the fundamental nature of knowledge, reality, and existence, often considered a theoretical basis of disciplines such as ethics, logic, and metaphysics.
Intellectual Parents
Parents who are characterized by or engage in activities that involve a high level of thinking and reasoning, often involving scholarly, professional, or creative pursuits.
Physiology
The science of how the body and its systems work, including biological and chemical processes.
Q10: Valence electrons are electrons located _.<br>A)in the
Q16: One of the most important correlates of
Q50: The first disorder unique to children and
Q59: The SI unit used to measure the
Q62: X-rays are generated by the nucleus during
Q63: The number of dots in the electron
Q68: A polar covalent bond is found in
Q81: The following is a decomposition reaction.<br>2KCl <img
Q92: The _ is the energy difference between
Q96: The name of the compound CuSO4 is