What is the IUPAC name for the following compound? 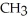 Cl
Cl 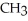 -
-  H -
H - 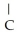 - CH = CH
- CH = CH 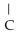

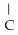
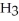
Definitions:
Resonance Energy
The difference in energy between the actual structure of a molecule and the energy of the most stable resonance structure, indicating stability due to resonance.
Stability
The tendency of a chemical compound to maintain its original state or resist decomposition under specific conditions.
S-trans Conformation
A spatial arrangement in which substituent groups are on opposite sides of a double bond or single bond in a chain, leading to a more extended structure.
Heats Of Hydrogenation
The change in enthalpy that occurs when a compound is hydrogenated, often used to measure the stability of double or triple bonds.
Q6: The name of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="The name
Q16: What is the IUPAC name for a
Q19: Given the two C++ array declarations:<br>int a[10],b[10];<br>You
Q20: The product of (-4)× (-5)is _.<br>A)-20<br>B)+20<br>C)-1<br>D)+1<br>E)0
Q24: Alkenes and alkynes are called unsaturated compounds
Q27: In the definition,double d[10] = {0.0};only d[0]
Q56: A carbohydrate that gives two molecules when
Q57: Amines act as weak acids by accepting
Q67: According to the IUPAC convention for chemical
Q94: Light-induced cis-trans isomerization is an important step