For the reaction NO2 (g)+ N2O(g)  3 NO(g)the equilibrium constant expression is:
3 NO(g)the equilibrium constant expression is: 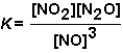
Definitions:
Basal Metabolic Rate
The number of calories required to keep your body functioning at rest, often referred to as BMR.
Settling Point
A theory suggesting that body weight is regulated at a predetermined or preferred level by a feedback control mechanism, which balances energy intake with energy expenditure.
Body Mass Index
A measurement calculated by dividing a person's weight in kilograms by the square of their height in meters, used to assess whether an individual has a healthy body weight for their height.
Close Relationships
Interpersonal relationships characterized by strong, deep, or close association between two or more individuals.
Q16: Bayes's theorem is based on:<br>A) conjecture.<br>B) experimental
Q27: A solution is prepared by dissolving 4.66
Q28: All neurons look alike and behave in
Q29: An axon's _ of firing determine(s)how it
Q31: Which of the following is an endergonic
Q35: Which of the following specific temperatures is
Q38: Goldstein and Gigerenzer (1999,2002)display the utility of
Q40: A is the antecedent,and B is the
Q56: Nonoxygenated hemoglobin is called _ .<br>A)deoxyhemoglobin<br>B)carboxylhemoglobin<br>C)carbaminohemoglobin<br>D)nitrosohemoglobin
Q84: Describe the specializations of each hemisphere of