During a Metabolic Pathway the Following Reaction Produced 3 G°' for the Hydrolysis of ATP Is -7
During a metabolic pathway the following reaction produced 3.00 moles of product.If G°' for the hydrolysis of ATP is -7.3 kcal/mol,how much energy would be involved? 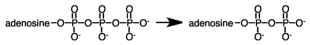
Definitions:
Grantor
In legal contexts, this refers to the individual or entity that creates a trust, conveys property, or grants a right.
Warranties of Title
Legal guarantees provided by a seller to a buyer that they have the rightful ownership of the item or property being sold and have the right to sell it.
Executes
Carries out or performs a task or action, often with precision and according to a plan or law.
Statute of Frauds
The Statute of Frauds is a legal concept requiring certain types of contracts to be written down and signed by all parties involved to be enforceable.
Q1: The products of the reaction of a
Q5: Which of the following groups are expected
Q11: All pyruvate has a similar fate,in that
Q12: A substance that when added to a
Q29: Complete <span class="ql-formula" data-value="\beta"><span class="katex"><span
Q35: Under saturation conditions,an enzyme-catalyzed reaction had a
Q67: Which of the following statements about DNA
Q71: Which of the following is correctly classified
Q72: Naturally occurring lithium (Li)consists of only two
Q80: Which fat-soluble vitamin is involved in the