Solved
During a Metabolic Pathway the Following Reaction Produced 3 G°' for the Hydrolysis of ATP Is -7
Multiple Choice
During a metabolic pathway the following reaction produced 3.00 moles of product.If G°' for the hydrolysis of ATP is -7.3 kcal/mol,how much energy would be involved? 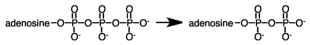
Definitions:
Related Questions
Q1: In the diagram of the citric acid
Q25: The accumulation of ketone bodies in the
Q27: Consider the figure shown below where four
Q35: How many neutrons are found in the
Q39: Glycolipids are abundant in which tissue?<br>A)muscle<br>B)brain<br>C)heart<br>D)liver
Q43: What units are used for protein,vitamins and
Q67: What rule,principle or law is used to
Q73: Using whole numbers,determine the molecular weight of
Q79: Waxes differ from fats and oils in
Q83: Hematuria is a condition where which elevated