Multiple Choice
How many mL of a 0.150 M solution of KOH would be required to titrate 25.0 mL of a 0.125 M solution of the following acid,which can be used as a standard in acid-base titrations? 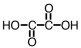
Definitions:
Related Questions
Q1: The predominant form of 1-propanamine at low
Q2: Which of the following is the substance
Q15: An isotope of a given element has
Q21: An alkaloid present in some cough syrups
Q28: The decay of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5752/.jpg" alt="The decay
Q38: A characteristic of alkynes is a region
Q46: Which term correctly describes the following reaction?
Q53: How can the solubility of alcohols be
Q70: Many commercials sell fiber supplements but you
Q80: A major source of aromatic compounds is