System 1 is 3.5 moles of a monatomic ideal gas held at a constant volume of 2.22 L as it undergoes a temperature increase.System 2 is 3.5 moles of a diatomic ideal gas held at a constant volume of 2.22 L as it undergoes a temperature increase equal to the temperature increase of System 1.The pressure increase for system 1 is 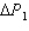 and for System 2 is
and for System 2 is 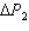 during the temperature increases.What is the ratio of
during the temperature increases.What is the ratio of 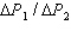 ?
?
Definitions:
Total Mastectomy
The complete surgical removal of one or both breasts, typically to treat or prevent breast cancer.
Blood Pressure Cuff
An apparatus for gauging blood pressure, which incorporates an expandable cuff for limiting blood circulation and a manometer for assessing the pressure.
Invasive Measurement
Techniques that require entry into the body, through skin or a body cavity, to obtain accurate data regarding health conditions or physiological functions.
Tympanic Thermometer
A device used to measure temperature by detecting the infrared heat from the ear drum.
Q1: A new nurse wants feedback from the
Q7: Two springs,each with spring constant 20 N/m,are
Q29: Which best expresses the value for the
Q42: Waves propagate at 8.0 m/s along a
Q42: An electron is released from rest at
Q44: A 3.00-g lead bullet is traveling
Q56: A rectangular steel plate with dimensions
Q58: Areas always have dimensions _ while volumes
Q62: Different units can be used for length:
Q69: A Carnot engine runs between a hot