System 1 is 3.5 moles of a monatomic ideal gas held at a constant volume of 2.22 L as it undergoes a temperature increase.System 2 is 3.5 moles of a diatomic ideal gas held at a constant volume of 2.22 L as it undergoes a temperature increase equal to the temperature increase of System 1.The pressure increase for system 1 is 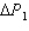 and for System 2 is
and for System 2 is 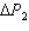 during the temperature increases.What is the ratio of
during the temperature increases.What is the ratio of 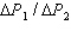 ?
?
Definitions:
Convertible Bond
A type of corporate bond that can be converted into a predetermined number of the company's equity shares at certain times during its life, usually at the discretion of the bondholder.
Conversion Premium
The additional cost over the current price that one pays to convert a convertible security into stock.
Lease Obligations
Liabilities representing the amount owed under lease agreements for the use of property, plant, or equipment.
Special-Use Trailers
Trailers designed for specific purposes or to carry specific cargo, not suitable for general freighting tasks.
Q6: The sulfur hexafluoride molecule consists of one
Q10: The nurse manager plans to send an
Q10: If the size of the charge value
Q16: Using a resistor and two different value
Q21: When current is flowing in a superconductor,which
Q31: Two moles of nitrogen gas are contained
Q33: Two equal charges,each Q,are separated by some
Q38: Suppose a power plant uses a Carnot
Q45: What is the current flowing through the
Q50: As a train starts from rest and