Starting with the expression for work 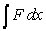 , one can change the look of the expression without changing what it represents by dividing the force F by the area it is applied over and multiplying the differential distance dx by that same area. Force divided by area is a pressure (P) , and area times the differential distance is a differential volume. Hence one can also describe work as
, one can change the look of the expression without changing what it represents by dividing the force F by the area it is applied over and multiplying the differential distance dx by that same area. Force divided by area is a pressure (P) , and area times the differential distance is a differential volume. Hence one can also describe work as 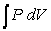 . A gas at constant temperature will change its pressure inversely to changes in volume,
. A gas at constant temperature will change its pressure inversely to changes in volume, 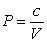 . If a sample of gas has a pressure of 1 atmosphere when its volume is 1 L, how much work does it do when it expands from 1 L to 3 L?
. If a sample of gas has a pressure of 1 atmosphere when its volume is 1 L, how much work does it do when it expands from 1 L to 3 L?
Definitions:
Assertive Communication
The practice of expressing one's views and wishes directly and respectfully, without being passively accepting or aggressively confrontational.
Lack of Participation
The absence or insufficient level of engagement or involvement in a particular activity or setting.
Preparation
The process of making ready or being made ready for use or consideration, involving planning and arrangement of resources or activities.
Verbal Communication
The conveyance of information or feelings through spoken words, encompassing elements like language, tone, and inflection for effective interaction.
Q2: Use the properties of logarithms to rewrite
Q11: Find the equation of the tangent line
Q11: Set up the integral for arc length
Q21: A certain producer determines that his cost
Q61: Determine the interval of convergence and the
Q62: Find the derivative of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5869/.jpg" alt="Find
Q73: Identify the graph and area of the
Q75: Find the derivative of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5869/.jpg" alt="Find
Q86: Estimate the error in the approximation of
Q117: Several useful integration formulas called reduction formulas