Suppose 100 mL of a 0.2 M aqueous solution of histidine is titrated with 0.4 M NaOH.Given that the pKa of the -COOH group,the -NH3+ group,and the -R group are approximately 1.80,9.33,and 6.04,respectively,sketch the titration curve. 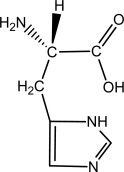 histidine
histidine
What are the approximate pKa values of the carboxylic acid group,the amine group,and the side group (if applicable)?
Definitions:
Interest Rate
The percentage of the principal amount charged by a lender to a borrower for the use of assets, typically expressed on an annual basis.
Compounded Semiannually
Interest on a loan or investment calculated twice a year and added to the principal so that subsequent interest is earned on the increased principal.
Annual Interest
The amount of interest payable every year on a loan or investment, typically expressed as a percentage of the principal amount.
High School Graduation
The completion of the required coursework and achievement of necessary credits for a student to officially finish high school and receive a diploma.
Q22: Which of the following refers to an
Q53: L-alanine has the molecular structure shown.Which of
Q103: Which of the following are NOT considered
Q117: Bombarding atoms with <span class="ql-formula"
Q120: Describe what is meant by the primary
Q145: Which of the following statements regarding enzymes
Q146: Which statement regarding amino acids found
Q146: Suppose 89.4% of a sample of radioactive
Q155: Iron (Fe)crystallizes as a body-centered unit cell
Q186: Complete and balance this nuclear equation