Using the bond enthalpy values below,the fuel value for octane is calculated as 44.0 kJ/g,which is close to its experimental value.Predict whether the fuel value of propyl-butylether,C7H16O,will be larger or smaller than that of octane,and calculate an estimate of its fuel value using the data provided.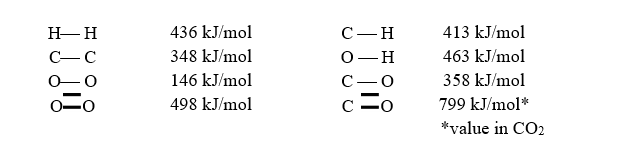
Definitions:
Traditional Marketing
Marketing techniques that utilize traditional channels such as print media, broadcasting, direct mail, and telephone to communicate a marketing message to consumers.
Indirect Competition
Competition between businesses offering different products or services that satisfy the same customer needs or desires.
Direct Competition
Companies or businesses that offer the same or similar products or services and target the same customer base, competing directly in the market.
Nonprofit Organizations
Nonprofit Organizations are entities that operate for the collective, public, or social benefit, rather than to generate profit for owners or investors.
Q9: A polymer consisting of monomers A and
Q23: A voltaic cell is constructed based on
Q44: Calculate E<sub>cell</sub> for an electrochemical cell
Q50: Electrochemical cell potentials can be used
Q60: The repeating unit of poly(ethylene glycol),a commercially
Q80: Which of the following is a carboxylic
Q87: The following reaction occurs in basic
Q101: Based on the information in the table
Q138: Write the names that the acronyms DNA
Q140: How many chelation sites and donor groups