Using the following data,determine the standard cell potential 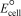 for the electrochemical cell constructed using the following reaction: Zn(s) + Pb2+(aq) Zn2+(aq) + Pb(s) . Half-reaction Standard reduction potential
for the electrochemical cell constructed using the following reaction: Zn(s) + Pb2+(aq) Zn2+(aq) + Pb(s) . Half-reaction Standard reduction potential
Zn2+(aq) + 2 e- Zn(s) -0.763
Pb2+(aq) + 2 e- Pb(s) -0.126
Definitions:
Wrong Medication
An error that involves administering or prescribing a medication that is not the correct one for the patient's condition or specifications.
Health Professions Act
Legislation designed to regulate the practice of health professions, protect public interest, and ensure competent and ethical practice among health professionals.
Anonymous Letter
A letter sent without disclosing the identity of the sender.
Q37: What is the chemical formula of aminetrichloroplatinate(II)?<br>A)[Pt<sub>2</sub>(NH<sub>3</sub>)Cl<sub>3</sub>]<sup>-</sup><br>B)[Pt(NH<sub>3</sub>)<sub>3</sub>Cl]<sup>-</sup><br>C)[Pt(NH<sub>3</sub>)Cl<sub>3</sub>]<sup>2</sup><sup>-</sup><br>D)[Pt(NH<sub>3</sub>)Cl<sub>3</sub>]<sup>+</sup><br>E)[Pt(NH<sub>3</sub>)Cl<sub>3</sub>]<sup>-</sup>
Q64: As the value of the crystal
Q73: Nickel ions,Ni<sup>2+</sup>,should have the greatest affinity for
Q99: Write a balanced chemical equation using skeleton
Q99: You decide to use your chemistry knowledge
Q135: Write the balanced chemical reaction showing the
Q140: Examine the three triglycerides shown. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6561/.jpg"
Q145: Calculate the packing efficiency of a body-centered
Q153: A phosphate buffer solution (25.00 mL sample)used
Q237: Sodium benzoate (NaC<sub>6</sub>H<sub>5</sub>COO)is a common food