Using the following data,determine 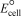 for the electrochemical cell constructed using the reaction of zinc and chromate ions under basic conditions.Unbalanced: Zn(s) + 2 CrO42-(aq) Zn(OH) 2(s) + Cr(OH) 3(s)
for the electrochemical cell constructed using the reaction of zinc and chromate ions under basic conditions.Unbalanced: Zn(s) + 2 CrO42-(aq) Zn(OH) 2(s) + Cr(OH) 3(s)
Half-reaction Standard reduction potential
Zn(OH) 2(s) + 2 e- Zn(s) + 2 OH-(aq) -1.25
CrO42-(aq) + 4 H2O(  ) + 3 e- Cr(OH) 3(s) + 5 OH-(aq) -0.13
) + 3 e- Cr(OH) 3(s) + 5 OH-(aq) -0.13
Definitions:
Development
The process of growth, progress, or improvement in a person, organization, or product.
Work Sampling
A technique used to estimate the proportion of time spent by workers on different tasks by observing them at random intervals.
Driving Abilities
the skills and capabilities needed to operate a vehicle safely and efficiently on the road.
Interviewers
Individuals who ask questions in various contexts, such as job interviews, surveys, or research, to gather information or assess qualifications.
Q1: Bromocresol green is yellow in its acidic
Q60: Suppose a 1.0 L solution containing
Q93: Describe the structural features of an
Q131: Which of the following statements regarding phospholipids
Q134: What is the name of the following
Q138: Describe the ccp and hcp crystal structures.Include
Q147: The C - O - C bonding
Q148: Suppose 100 mL of a 0.2 M
Q174: Acid-base indicators need to have very intense
Q222: What is the percent dissociation of