Based on the information in the table of standard reduction potentials below,what is the standard cell potential for an electrochemical cell that has iron (Fe) and magnesium (Mg) electrodes immersed in 1M Fe3+ and Mg2+ solutions? Also,identify the cathode. 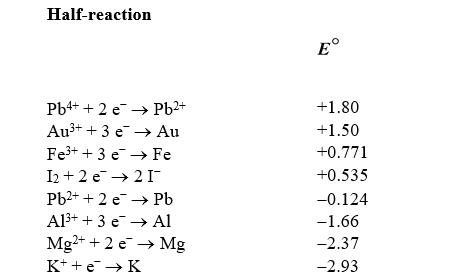
Definitions:
Reinvested Profit
Earnings that a company plows back into itself to fund growth, debt repayment, or asset purchases rather than distributing to shareholders as dividends.
Income Statement
A financial statement that shows a company’s revenues and expenses over a specific period, providing insight into its profitability.
Cash Flow
The total amount of money being transferred into and out of a business, especially affecting liquidity.
Reported Profits
These are the earnings a company officially announces to the public and regulatory bodies, reflecting its performance over a specific period.
Q17: A NiMH battery uses _ as the
Q22: Suppose 10.0 mL of 2.00 M
Q36: Consider the following aqueous equilibrium for
Q70: Structural forms of an element in which
Q73: Phosphoric acid is a triprotic acid
Q95: Which sketch best represents the qualitative molecular
Q101: How many structural isomers does xylenol,(CH<sub>3</sub>)<sub>2</sub>C<sub>6</sub>H<sub>3</sub>OH,have?<br>A)None-i.e.,there is
Q109: In the following reaction,which species is
Q110: This molecule is _ <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6561/.jpg" alt="This
Q148: The two types of closest-packed lattices are