A concentration cell is constructed by using the same half-reaction for both the cathode and anode.What is the value of standard cell potential, 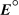 ,for a concentration cell that combines a silver anode in contact with 0.10 M silver nitrate and a silver cathode in contact with 0.00003 M silver nitrate? (
,for a concentration cell that combines a silver anode in contact with 0.10 M silver nitrate and a silver cathode in contact with 0.00003 M silver nitrate? ( 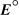 = +0.80 V for Ag/Ag+)
= +0.80 V for Ag/Ag+)
Definitions:
Observing And Measuring
The process of collecting data about phenomena or behaviors through direct observation and using tools to quantify the observations.
Empirically Tested
Evaluated or tested through observation or experiment rather than theory or pure logic.
Variables
Characteristics of behavior or experience that can be measured or described by a numeric scale.
Hypothesis
A proposed explanation made on the basis of limited evidence as a starting point for further investigation.
Q21: Which of the following metal ions can
Q30: What is the degree of unsaturation of
Q40: Of C,Si,Ge,and Sn,which elements are semiconductors?<br>A)C and
Q49: The Nernst equation can be used to
Q62: Which of the following pairs of molecules
Q102: A voltaic cell is constructed based on
Q140: How many chelation sites and donor groups
Q161: In antifluorite structures,_<br>A)the cations are smaller than
Q176: Draw a few subunits of the
Q200: What is the actual concentration of