Using the following data,determine 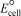 for the electrochemical cell constructed using the reaction of nitrous oxide and oxalic acid under acidic conditions.Unbalanced: N2O(g) + H2C2O4(s) 2 CO2(g) + N2(g) + H2O(
for the electrochemical cell constructed using the reaction of nitrous oxide and oxalic acid under acidic conditions.Unbalanced: N2O(g) + H2C2O4(s) 2 CO2(g) + N2(g) + H2O(  )
)
Half-reaction Standard reduction potential
2 CO2(g) + 2 H+(aq) + 2 e- H2C2O4(s) -0.49
N2O(g) + 2 H+(aq) + 2 e- N2(g) + H2O(  ) +1.78
) +1.78
Definitions:
Model Programs
These are structured programs designed as exemplary frameworks or best practice guides to address specific issues, often developed based on research and evidence.
Delinquency
Minor crime, especially that committed by young people, involving behaviors that violate legal and social standards.
Congressperson
An elected member of the legislative branch of the United States of America, serving in the United States Congress, which consists of the Senate and the House of Representatives.
Head Start
A program in the United States that provides comprehensive education, health, nutrition, and parent involvement services to low-income children and their families.
Q18: Which of the following groups,a-d,consist of salts
Q38: What is the oxidation state of cobalt
Q71: Which of the following statements regarding chemical
Q80: A solution that contains a weak acid
Q93: Electrical and thermal conductivity in metals _<br>A)is
Q107: The structure of the acetylacetonate ligand is
Q118: What is the formula of pentaamineiodorhodium(III)iodide?
Q159: Explain why some salts produce neutral solutions
Q162: Do carbon atoms in aromatic hydrocarbon molecules
Q184: Which expression defines the autoionization constant for