What is the standard cell potential for a voltaic cell using the Pb2+/Pb and Mg2+/Mg half-reactions? Which metal is the cathode?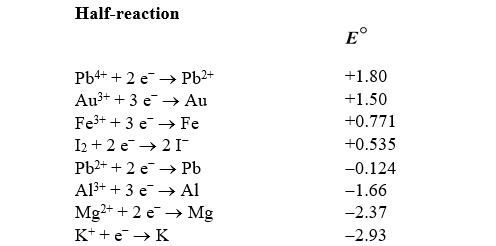
Definitions:
Sodium
A chemical element (Na) that is a soft, silver-white, highly reactive metal belonging to the alkali metal group, essential for humans and animals in small amounts.
Acid-Base Balance
The mechanism by which the body maintains its normal pH level, ensuring optimal functioning of cells and organs.
Fluid Balance
The maintenance of the correct amount of fluid in the body, crucial for ensuring that all cells and organs function effectively.
Clear-Liquid Diet
A diet consisting only of clear liquids like water, broth, and certain juices, often recommended before medical tests or surgery.
Q17: Which of the following is a strong
Q47: Three acids found in foods are lactic
Q47: Electrolytic processes are used in a variety
Q67: In addition to carbon and iron,stainless steel
Q89: Which of the following statements regarding methanol
Q98: Pure solid metals _<br>A)do not crystallize.<br>B)are amorphous.<br>C)often
Q110: A face-centered cubic unit cell has a(n)_
Q143: Lead pipes were used at one
Q144: When Si is doped with P,it produces
Q167: What is the systematic name of the