Using the following data,determine the standard cell potential 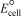 for the electrochemical cell constructed based on the following unbalanced reaction expression: Al(s) + Cr3+(aq) Al3+(g) + Cr2+(aq) .
for the electrochemical cell constructed based on the following unbalanced reaction expression: Al(s) + Cr3+(aq) Al3+(g) + Cr2+(aq) .
Half-reaction Standard reduction potential
Al3+(aq) + 3 e- Al(s) -1.66
Cr3+(aq) + e- Cr2+(aq) -0.41
Definitions:
Referents
The actual objects, actions, or concepts that words or symbols represent or signify.
Subcontrary
In traditional logic, a relationship between two statements where both can be true but both cannot be false.
Space Cowboy
A colloquial, imaginative term often used to describe someone who explores or navigates the domain of outer space in a freewheeling, adventurous manner, popularized by science fiction and music.
Proposition
In logic, a statement or assertion that expresses a judgment or opinion which can be either true or false.
Q22: What is the pH of a
Q35: The face-centered cubic structure is also known
Q42: In the following reaction,which species is
Q49: Band theory of bonding in solids _<br>A)is
Q70: What is the cell potential of
Q83: What reaction occurs as sodium hydroxide
Q154: Without working out all the structures,which has
Q177: Molybdenum adopts a bcc structure with a
Q196: In the Brønsted-Lowry definition of acids and
Q227: Which of the following would be