Using the following data,determine 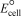 for the electrochemical cell constructed using the reaction of zinc and chromate ions under basic conditions.Unbalanced: Zn(s) + 2 CrO42-(aq) Zn(OH) 2(s) + Cr(OH) 3(s)
for the electrochemical cell constructed using the reaction of zinc and chromate ions under basic conditions.Unbalanced: Zn(s) + 2 CrO42-(aq) Zn(OH) 2(s) + Cr(OH) 3(s)
Half-reaction Standard reduction potential
Zn(OH) 2(s) + 2 e- Zn(s) + 2 OH-(aq) -1.25
CrO42-(aq) + 4 H2O(  ) + 3 e- Cr(OH) 3(s) + 5 OH-(aq) -0.13
) + 3 e- Cr(OH) 3(s) + 5 OH-(aq) -0.13
Definitions:
Pictorial Sketches
Drawings that represent three-dimensional objects on a two-dimensional surface, providing a realistic view and helping in visualizing the object.
Perspective
A technique in drawing or painting to represent three-dimensional objects on a two-dimensional surface, depicting depth and position relative to each other.
Isometric
A method of drawing or projection where the object is shown in three dimensions in a single view, with the axes typically inclined at equal angles to provide depth.
Hidden Lines
Dashed lines used in technical drawings to indicate edges or surfaces that are not directly visible from the current viewpoint.
Q30: The molar solubility,s,for Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> is written as
Q54: If a face-centered cubic unit cell
Q56: Oxidation refers to _<br>A)an increase in oxidation
Q89: Reduction refers to _<br>A)loss of mass.<br>B)an increase
Q91: Transition metals with _ tend to form
Q100: Which one of the following is NOT
Q108: What is NOT a correct formula for
Q117: What must be true about the
Q129: What monomer is produced when starch is
Q195: What is the pH of a solution