Based on the information in the table of standard reduction potentials below,what is the standard cell potential for an electrochemical cell that has iron (Fe) and magnesium (Mg) electrodes immersed in 1M Fe3+ and Mg2+ solutions? Also,identify the cathode. 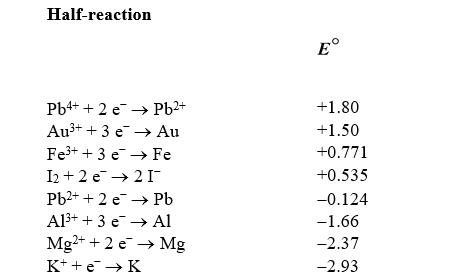
Definitions:
Reasonable Care
The level of carefulness and attention that a reasonably wise and practical individual would apply under comparable conditions.
Landlord
The owner of a property who leases it to a tenant under a rental agreement, responsible for maintaining the property and adhering to the lease terms.
Tenants
Tenants are individuals who occupy land or property rented from a landlord.
Common Areas
Spaces within a building or property that are available for use by all occupants or members, such as lobbies, corridors, and recreational facilities.
Q9: What determines whether a transition metal ion
Q21: Briefly explain why the density of low-density
Q24: A concentration cell is constructed by using
Q56: Which base is NOT present in RNA?<br>A)adenine<br>B)cytosine<br>C)guanine<br>D)thymine<br>E)uracil
Q58: What does it mean to describe an
Q71: Which of the following statements regarding chemical
Q74: Firing of a kaolinite clay object to
Q152: For each of the numbered bonds in
Q153: A phosphate buffer solution (25.00 mL sample)used
Q164: Which of the following molecular structures is