Calculate 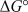 for an electrochemical cell reaction that occurs under basic aqueous conditions based on the following two half-reactions for which the standard reduction potentials are given.Use the smallest whole-number coefficients possible when balancing the overall reaction.
for an electrochemical cell reaction that occurs under basic aqueous conditions based on the following two half-reactions for which the standard reduction potentials are given.Use the smallest whole-number coefficients possible when balancing the overall reaction.
Cd(OH) 2 + 2 e- Cd + 2 OH- -0.824 V
NiO(OH) + H2O + e- Ni(OH) 2 + OH- +1.32 V
Definitions:
Economic Efficiency
The optimal allocation of resources where output is maximized for a given set of inputs, or the input cost is minimized for a certain level of output.
Unions
Organizations formed by workers from related fields that work for the collective welfare of their members, focusing on negotiating wage rates, work practices, and benefits.
U.S. Labor Law
The body of laws, regulations, and judicial decisions concerning the rights of workers, labor unions, and employers in the United States.
Schools of Thought
Various intellectual movements or perspectives within a discipline, each with its own distinct theory and methodology.
Q32: Gold has a face-centered cubic structure with
Q32: The solubility of PbBr<sub>2</sub> is 0.427
Q40: Given the following K<sub>a</sub> values,which of the
Q42: Determine the number of carbons in the
Q47: Describe what is meant by the quaternary
Q56: Oxidation refers to _<br>A)an increase in oxidation
Q63: What is the equilibrium concentration of
Q80: Iron exhibits polymorphism,the ability of a
Q125: Which of the following statements regarding MTBE,methyl
Q207: Which of the following is a strong