A concentration cell is constructed by using the same half-reaction for both the cathode and anode.What is the value of standard cell potential, 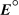 ,for a concentration cell that combines a silver anode in contact with 0.10 M silver nitrate and a silver cathode in contact with 0.00003 M silver nitrate? (
,for a concentration cell that combines a silver anode in contact with 0.10 M silver nitrate and a silver cathode in contact with 0.00003 M silver nitrate? ( 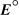 = +0.80 V for Ag/Ag+)
= +0.80 V for Ag/Ag+)
Definitions:
Units Produced
The total quantity of items manufactured by a company within a specific period, indicating production volume.
Distorted Product Cost
Occurs when the assigned costs of a product do not accurately reflect the actual resources consumed by the product.
Poor Decisions
Choices made with lack of judgment or information that result in unfavorable outcomes.
Volume-related Measures
Metrics that evaluate performance, capacity, or financial results based on the volume of activities, production, or transactions.
Q4: A 0.025 L sample of oven cleaner
Q29: Magnesium oxide adopts a rock salt structure
Q38: Which one of the following items does
Q42: Determine the number of carbons in the
Q47: Describe what is meant by the quaternary
Q56: A solution containing the ion CoF<sub>6</sub><sup>3</sup><sup>-</sup>
Q57: Aluminum is resistant to corrosion because of
Q98: Define the term Lewis base and provide
Q135: What is the correct formula for sodium
Q152: What is the actual concentration of