Calculate the equilibrium constant for the oxidation of bromine by ozone at 298 K. O3(g) + 2 H+(aq) + 2 e- H2O(  )
)
E° = +2.08 V
BrO3-(g) + 6 H+(aq) + 5 e- 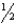 Br2(s) + 3 H2O(
Br2(s) + 3 H2O(  )
)
E° = +1.52 V
Definitions:
Health Care Spending
The total amount of money spent on health care services and products in a country or region within a specified period.
Private Health Insurance
Health insurance coverage provided by private entities rather than the government to cover medical expenses.
Wage Controls
Governmental policies or regulations aimed at setting or capping the levels of wages within an economy.
Q13: Which of the following are held together
Q32: Which of the following molecules has the
Q71: A molecule with the formula C<sub>7</sub>H<sub>14</sub> was
Q71: In a two-component alloy,the more abundant metal
Q96: The acid ionization equilibrium constant,K<sub>a</sub>,describes which
Q100: Aluminum (Al)has a density of 2.70 g/cm<sup>3</sup>
Q127: A chemical equilibrium 2<sub> </sub>A
Q128: What type of amine is adrenaline,which is
Q167: The pK<sub>a</sub> of a weak acid was
Q171: Which functional group is found in the