Calculate the equilibrium constant for the spontaneous redox reaction at 298 K that would occur using the following two half-reactions.
ClO3-(aq) + 6 H+(aq) + 5 e- 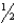 Cl2(g) + 3 H2O(
Cl2(g) + 3 H2O( 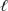 ) E° = +1.47 V
) E° = +1.47 V
HClO(aq) + H+(aq) + e- 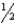 Cl2(s) + H2O(
Cl2(s) + H2O( 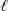 )
)
E° = +1.63 V
Definitions:
Temporary Accounts
Accounts that are closed at the end of each accounting period, including revenue, expense, and dividend accounts.
Adjusting Entry
A journal entry made at the end of an accounting period to update account balances before preparing financial statements.
Unearned Service Revenue
Represents payments received for services that have not yet been performed or delivered, classified as a liability on the balance sheet.
Advance Payment
Money paid before it is due or for goods or services before they are received.
Q5: An electronic device requires two 1.50-V
Q16: If a face-centered cubic unit cell
Q21: Solder is a fusible metal alloy used
Q32: Which of the following molecules has the
Q37: Which of the following is the ketone
Q42: Why are ceramic superconductors not currently practical
Q100: Nylon is a _<br>A)polyester.<br>B)polyamide.<br>C)polyaminoacid.<br>D)polyaromatic.<br>E)polyether.
Q164: Which of the following molecular structures is
Q170: The magnitude of the charge on
Q172: An electrochemical cell at 298 K