The oxidation of hydrogen by oxygen is one of the most-used reactions in fuel-cell technology.The overall reaction,which is given below,has a G° value of -237 kJ/mol.What is the standard cell potential for this fuel cell? H2(g) + 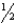 O2(g) H2O(
O2(g) H2O(  )
)
Definitions:
Q4: How many stereoisomers are there for the
Q9: A polymer consisting of monomers A and
Q9: Copper metal is purified by electrolysis.How much
Q40: Which of the following is NOT likely
Q93: Hexane and 2-methylpentane are _<br>A)enantiomers.<br>B)diastereomers.<br>C)chain isomers.<br>D)functional isomers.<br>E)positional
Q116: What is the equilibrium concentration of
Q120: Of an unknown compound containing only carbon
Q123: Which of the following is the
Q164: Gold (Au)has a face-centered cubic structure with
Q235: What is the pH of a