Permanganate ions can oxidize sulfite in basic solution according to the following equation.The relevant standard reduction potentials are 0.59 V for the manganese half-reaction and -0.92 V for the sulfur half-reaction.Determine the cell potential for the reaction at 298 K with the concentrations in the table.
2 MnO4-(aq)+ 3 SO32-(aq)+ H2O( 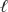 ) 2 MnO2(s)+ 3 SO42-(aq)+ 2 OH-(aq)
) 2 MnO2(s)+ 3 SO42-(aq)+ 2 OH-(aq)
Definitions:
Sensory Memory
The memory of an event that is recorded by sensory neurons that lasts for a very brief time after the event ends.
Iconic Memory
The visual memory of an event stored in the sensory register.
Echoic Memory
The auditory memory of an event stored in the sensory register.
Serial Position Effect
The faster learning and greater recall of items at the beginning and end of a list rather than at the middle of the list.
Q24: A concentration cell is constructed by using
Q32: The solubility of PbBr<sub>2</sub> is 0.427
Q39: Which is the correct formula for pentaamminecyanocobaltate(III)chloride?<br>A)[Co(NH<sub>3</sub>)<sub>5</sub>CN]Cl<sub>2</sub><br>B)[Co(NH<sub>3</sub>)<sub>5</sub>CN]Cl<sub>3</sub><br>C)[Co(NH<sub>3</sub>)<sub>5</sub>Cl](CN)<sub>2</sub><br>D)[Co(NH<sub>3</sub>)<sub>5</sub>CNO]Cl<sub>2</sub><br>E)[Co(NH<sub>3</sub>)<sub>5</sub>CNO]Cl<sub>3</sub>
Q43: A tetrahedral hole in a crystal lattice
Q51: Aluminum (Al)crystallizes as a face-centered unit cell
Q55: What reaction occurs as a hydrochloric
Q74: How many unpaired electrons are there in
Q93: Which of the following metal hydroxides is
Q133: A Lewis base is any species capable
Q167: Does pH have an effect on