Given the following data,calculate the approximate value of the equilibrium constant at 298 K for the reaction,Ni(NH3) 62+(aq) + 3 en(aq) Ni(en) 32+(aq) + 6 NH3(aq) ,where en represents ethylenediamine.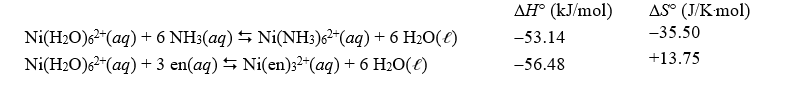
Definitions:
Q6: Which of the following molecules has enantiomeric
Q33: What is the molar solubility of
Q87: The following reaction occurs in basic
Q91: What are the similarities and differences between
Q99: You decide to use your chemistry knowledge
Q139: For the chemical equilibrium aA +
Q170: The solubility product for Ag<sub>3</sub>PO<sub>4</sub> is written
Q176: Aqueous solutions of _ are basic.<br>A)KF<br>B)NaCl<br>C)KBr<br>D)NaI<br>E)KI
Q180: Sodium hypochlorite (NaOCl,74.44 g/mol)is a common ingredient
Q205: Effects that stabilize the conjugate base of