Phosphoric acid is a triprotic acid in water,ionizing in the following sequential steps: H3PO4 + H2O H2PO4- + H3O+ 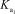 H2PO4- + H2O HPO42- + H3O+
H2PO4- + H2O HPO42- + H3O+ 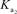 HPO42- + H2O PO43- + H3O+
HPO42- + H2O PO43- + H3O+
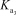 What is the appropriate Kb expression for a sodium phosphate solution?
What is the appropriate Kb expression for a sodium phosphate solution?
Definitions:
Direct Labor Standard
A predefined measure of the amount of labor time that is considered necessary to produce one unit of output.
Cost Standards
Predetermined costs that serve as a benchmark for evaluating the actual performance and budgeting of a company.
Past Cost Data
Historical financial information on the costs associated with production, operations, or projects, used for planning and analysis.
Technology
The application of scientific knowledge for practical purposes, especially in industry.
Q23: A voltaic cell is constructed based on
Q46: Which of the following metal ions can
Q74: Which of the following statements regarding the
Q74: How many unpaired electrons are there in
Q81: Which statement regarding battery-powered electric cars is
Q85: The standard cell potential for the
Q106: Determine the molar concentration of Ag<sup>+</sup>(aq)ions
Q130: The following figure shows Arrhenius plots for
Q167: For the reaction 2 A +
Q189: The linear form of the Arrhenius equation