Multiple Choice
Phosphoric acid is a triprotic acid in water,ionizing in the following sequential steps: H3PO4 + H2O F10 H2PO4- + H3O+ 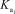 = 7.11 *10-3
= 7.11 *10-3
H2PO4- + H2O HPO42- + H3O+
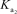 = 6.32 *10-8
= 6.32 *10-8
HPO42- + H2O PO43- + H3O+
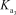 = 4.5 *10 - 13
= 4.5 *10 - 13
What is the pH of a 0.20 M H3PO4 solution?
Definitions:
Related Questions
Q16: Manganese(IV)oxide and chromium(III)hydroxide can react under
Q41: Write the expression for the reaction
Q87: When [H<sup>+</sup>] = 4.0 <span
Q98: The reaction of NO gas with
Q100: Which one of the following is NOT
Q113: Which of the following salts,when dissolved in
Q123: Briefly explain the features of a pseudo-first-order
Q123: What is the likely unit cell for
Q146: Which of the following statements regarding reduction
Q148: Consider the equilibrium A + B