Arsenic acid is a triprotic acid in water,ionizing in the following sequential steps:
H3AsO4 + H2O H2AsO4- + H3O+
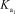 = 5.5 *10-3
= 5.5 *10-3
H2AsO4- + H2O HAsO42- + H3O+
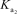 = 1.7 *10-7
= 1.7 *10-7
HAsO42- + H2O AsO43- + H3O+
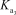 = 5.1 *10-12
= 5.1 *10-12
What is the approximate pH of a 0.80 M H3AsO4 solution?
Definitions:
Stimuli
External factors or changes in the environment that affect the senses and can elicit responses from an organism.
Determinism
The philosophical belief that all events, including moral choices, are determined completely by previously existing causes, implying that free will is an illusion.
Mental Terms
Language or vocabulary related to psychological states, processes, disorders, or treatments.
B. F. Skinner
An American psychologist and behaviorist known for his work in developing the theory of operant conditioning, which uses consequences to modify the occurrence and form of behavior.
Q3: Bubbles will form on wires attached to
Q18: For the rate law Rate = k[A]<sup>3/2</sup>[B],the
Q26: Which of the following is a polydentate
Q108: Write the cell diagram for the
Q129: A proposed mechanism for the reduction
Q143: A catalyst that is in a different
Q145: A ligand is any _ forming a
Q165: Which of the following describes a reaction
Q173: Given the following data for the
Q212: Phenylephrine (PE; see the structure below)is a