For the equilibrium 2 ClO(g) Cl2O2(g) ,Kc = 4.96 1011 at 253 K.What is Kc for the equilibrium 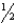 Cl2O2(g) ClO(g) ?
Cl2O2(g) ClO(g) ?
Definitions:
Marginal Product
The additional output resulting from one more unit of a given input, holding all other inputs constant.
Average Product
The output per unit of input, measured by dividing the total product by the quantity of input.
Average Product
The output per unit of input, calculated by dividing total production by the number of units of input.
Marginal Product
The additional output that is produced by adding one more unit of a particular input, keeping other inputs constant.
Q38: What is the oxidation state of cobalt
Q49: Given the following data,determine the molar free
Q65: If the rate of formation of
Q87: When [H<sup>+</sup>] = 4.0 <span
Q90: Consider a closed container containing a 1.00
Q114: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q115: Given the following data,determine the order
Q129: What is the percent ionization of 0.52
Q163: Which of the following is NOT important
Q193: Which one of the following is