For the equilibrium H2O(g) + CH4(g) CO(g) + 3 H2(g) ,Kp = 1.61 10-5 at 1400 K.At a given point,the partial pressures of the gases are 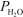 = 0.264 atm,
= 0.264 atm, 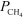 = 0.126 atm,
= 0.126 atm, 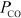 = 0.0382 atm,and
= 0.0382 atm,and 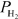 = 0.0974 atm.Which of the following statements is true?
= 0.0974 atm.Which of the following statements is true?
Definitions:
Depreciation Expense
Depreciation expense is the allocation of the cost of a tangible asset over its service life, reflecting the decrease in its value over time.
Book Value
The net value of a company's assets minus its liabilities, often used as an estimate of an asset's value if it were to be liquidated.
Accrued Property Taxes
Property taxes that have been incurred but not yet paid, typically recorded as a liability on the balance sheet.
Cost of Land
The purchase price and all associated costs, such as legal fees and surveying, of acquiring land for business use.
Q11: For the rate law Rate = k[A][B]<sup>1/2</sup>,the
Q12: Using the thermodynamic data below,determine the
Q13: Smog created by the interaction of sunlight
Q33: For the rate law Rate = k[A]<sup>1/2</sup>[B],the
Q46: A reaction is at equilibrium at
Q47: Given the following data,determine the rate
Q68: The entropy of vaporization of water is
Q121: For the rate law Rate = k[A]<sup>1/2</sup>[B],the
Q123: Estimate the standard molar entropy of
Q149: The enthalpy and entropy of vaporization