There are many reactions involving nitrogen,oxygen,and nitrogen oxides at high temperatures.At 1000.0 K,the following reactions have the given equilibrium constants: N2O4(g) 2 NO2(g) K1 = 1.5 106 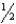 N2(g) + O2(g) NO2(g) K2 = 1.2 10-5
N2(g) + O2(g) NO2(g) K2 = 1.2 10-5
Calculate the theoretical value of the equilibrium constant for N2(g) + 2 O2(g) N2O4(g) .
Definitions:
Internal Control Steps
Procedures and measures adopted by a company to safeguard its assets, ensure financial report accuracy, and promote operational efficiency.
Receiving Report
A document used to record the receipt of goods from suppliers, detailing the quantities and condition of items delivered.
Reverse Order
A method of arranging or presenting items so that the last becomes first and the first last.
Last-In, First-Out
An inventory valuation method where the costs of the most recently produced or purchased items are the first to be expensed.
Q2: Which of the following occurs when reactants
Q10: If Q for a reaction is greater
Q29: The standard molar enthalpy fusion for
Q52: The rate of disappearance of HI
Q65: In evaluating the pH of an aqueous
Q95: Consider the freezing of water at
Q108: Write the cell diagram for the
Q153: For the coordination compound K<sub>3</sub>[Fe(CN)<sub>6</sub>],identify,in order,the charge
Q177: Suppose a 1.0 L solution containing
Q213: What is the pH of a