For the equilibrium H2O(g) + CH4(g) CO(g) + 3 H2(g) ,Kp = 1.61 10-5 at 1400 K.At a given point,the partial pressures of the gases are 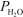 = 0.264 atm,
= 0.264 atm, 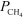 = 0.126 atm,
= 0.126 atm, 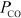 = 0.0382 atm,and
= 0.0382 atm,and 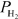 = 0.0974 atm.Which of the following statements is true?
= 0.0974 atm.Which of the following statements is true?
Definitions:
Predators
Animals that hunt, kill, and consume other organisms for food.
Invasive Species
Non-native organisms that spread rapidly in new environments, often causing harm to native species, human health, and economies.
Generalists
Organisms that have a broad niche and can thrive in a wide variety of environmental conditions and can make use of a variety of different resources.
Species Diversity
The variety and abundance of different species within a given area, contributing to the complexity and resilience of ecosystems.
Q2: Approximately how many times faster or
Q37: Consider the following equilibrium: CO(g)+ 3
Q45: The observed rate law for the
Q50: What is the name of [Fe(NH<sub>3</sub>)<sub>5</sub>Br]Cl<sub>2</sub>?
Q60: Suppose a 1.0 L solution containing
Q62: Hydrogen iodide can theoretically be made
Q105: If the average rate of reaction
Q113: Which of the following salts,when dissolved in
Q116: What is the equilibrium concentration of
Q230: Write the reaction and equilibrium constant that