For the equilibrium (CH3) 3CCl(g) (CH3) 2C = CH2(g) + HCl(g) ,Kp = 3.45 at 500 K.At a given point,the partial pressures of the gases are 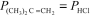 = 0.810 atm and
= 0.810 atm and 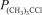 = 0.190 atm.Which of the following statements is true?
= 0.190 atm.Which of the following statements is true?
Definitions:
Price Paid
The amount of money exchanged for the acquisition of a good, service, or asset.
Original Issue Discount
The difference between the face value of a bond and its offering price when the bond is issued at a lower price.
Semiannual Coupon
A fixed income security feature that represents the payment of interest to bondholders twice a year.
Par Value
The nominal or face value assigned to a security by the issuer, which may differ from its market value.
Q5: Consider the equilibrium,Cl<sub>2</sub>(g)+ 2 NO(g) <span
Q16: A sealed tube containing an equilibrium
Q23: Define the term Lewis acid and provide
Q57: Which of the following square planar complexes
Q102: The oxidation of iodide by peroxodisulfate,S<sub>2</sub>O<sub>8</sub><sup>2-</sup>(aq)+
Q128: Considering the tabulated values for the thermodynamic
Q129: What is the percent ionization of 0.52
Q143: The decomposition of NOCl to form
Q153: Chromium often is electroplated on other metals
Q184: Which expression defines the autoionization constant for